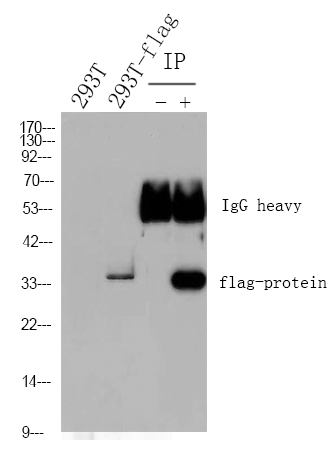
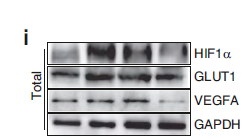
主要信息
Target
Amyloid-β
Host Species
Rabbit
Reactivity
Human, Mouse, Rat
Applications
WB, IHC, IF, ELISA
MW
117kD (Observed)
Conjugate/Modification
Unmodified

货号: YT0226
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
详细信息
推荐稀释比
WB 1:500-1:2000; IHC 1:100-1:300; IF 1:200-1:1000; ELISA 1:40000; Not yet tested in other applications.
组成
Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
特异性
Amyloid-β Polyclonal Antibody detects endogenous levels of Amyloid-β protein.
纯化工艺
The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
储存
-15°C to -25°C/1 year(Do not lower than -25°C)
浓度
1 mg/ml
实测条带
117kD
修饰
Unmodified
克隆性
Polyclonal
同种型
IgG
相关产品
抗原&靶点信息
免疫原:
The antiserum was produced against synthesized peptide derived from human APP. AA range:711-760
展开内容
特异性:
Amyloid-β Polyclonal Antibody detects endogenous levels of Amyloid-β protein.
展开内容
基因名称:
APP
展开内容
蛋白名称:
Amyloid beta A4 protein, Amyloid-β, Aβ
展开内容
别名:
APP ;
A4 ;
AD1 ;
Amyloid beta A4 protein ;
ABPP ;
APPI ;
APP ;
Alzheimer disease amyloid protein ;
Cerebral vascular amyloid peptide ;
CVAP ;
PreA4 ;
Protease nexin-II ;
PN-II
A4 ;
AD1 ;
Amyloid beta A4 protein ;
ABPP ;
APPI ;
APP ;
Alzheimer disease amyloid protein ;
Cerebral vascular amyloid peptide ;
CVAP ;
PreA4 ;
Protease nexin-II ;
PN-II
展开内容
背景:
This gene encodes a cell surface receptor and transmembrane precursor protein that is cleaved by secretases to form a number of peptides. Some of these peptides are secreted and can bind to the acetyltransferase complex APBB1/TIP60 to promote transcriptional activation, while others form the protein basis of the amyloid plaques found in the brains of patients with Alzheimer disease. In addition, two of the peptides are antimicrobial peptides, having been shown to have bacteriocidal and antifungal activities. Mutations in this gene have been implicated in autosomal dominant Alzheimer disease and cerebroarterial amyloidosis (cerebral amyloid angiopathy). Multiple transcript variants encoding several different isoforms have been found for this gene. [provided by RefSeq, Aug 2014],
展开内容
功能:
Alternative products:Additional isoforms seem to exist. Experimental confirmation may be lacking for some isoforms,Disease:Defects in APP are the cause of Alzheimer disease type 1 (AD1) [MIM:104300]. AD1 is a familial early-onset form of Alzheimer disease. It can be associated with cerebral amyloid angiopathy. Alzheimer disease is a neurodegenerative disorder characterized by progressive dementia, loss of cognitve abilities, and deposition of fibrillar amyloid proteins as intraneuronal neurofibrillary tangles, extracellular amyloid plaques and vascular amyloid deposits. The major constituent of these plaques is the neurotoxic amyloid-beta-APP 40-42 peptide (s), derived proteolytically from the transmembrane precursor protein APP by sequential secretase processing. The cytotoxic C-terminal fragments (CTFs) and the caspase-cleaved products such as C31 derived from APP, are also implicated in neuronal death.,Disease:Defects in APP are the cause of amyloidosis cerebroarterial Dutch type (AMYLCAD) [MIM:605714]; also known as hereditary cerebral hemorrhage with amyloidosis Dutch type (HCHWAD). AMYLCAD is a hereditary localized amyloidosis due to amyloid-beta A4 peptide(s) deposition in the cerebral vessels. Beta-APP40 is the predominant form of cerebrovascular amyloid. Amyloid is not found outside the nervous system. The principal clinical characteristics are recurrent cerebral and cerebellar hemorrhages, recurrent strokes, cerebral ischemia, cerebral infarction, and progressive mental deterioration. Onset of the disease is in middle age (44 to 60 years). Patients develop cerebral hemorrhage because of the severe cerebral amyloid angiopathy. Parenchymal amyloid deposits are rare and largely in the form of pre-amyloid lesions or diffuse plaque-like structures. They are Congo red negative and lack the dense amyloid cores commonly present in Alzheimer disease.,Disease:Defects in APP are the cause of amyloidosis cerebroarterial Iowa type (AMYLCAIW) [MIM:605714]. AMYLCAIW is a hereditary amyloidosis due to amyloid-beta A4 peptide(s) deposition. Patients have progressive aphasic dementia, leukoencephalopathy, and occipital calcifications.,Disease:Defects in APP are the cause of amyloidosis cerebroarterial Italian type (AMYLCAIT) [MIM:605714]. AMYLCAIT is a hereditary localized amyloidosis due to amyloid-beta A4 peptide(s) deposition in the cerebral vessels, resulting in cerebral amyloid angiopathy. Amyloid is not found outside the nervous system. It is a condition very similar to AMYLCAD, but the clinical course is less severe. Patients manifest mild cognitive decline, recurrent strokes, and epilepsy in some cases. There are extensive amyloid deposits in leptomeningeal and cortical vessels and, to a lesser extent, in the neuropil of the cerebral cortex, in the absence of neurofibrillary tangles.,Domain:The basolateral sorting signal (BaSS) is required for sorting of membrane proteins to the basolateral surface of epithelial cells.,Domain:The NPXY sequence motif found in many tyrosine-phosphorylated proteins is required for the specific binding of the PID domain. However, additional amino acids either N- or C-terminal to the NPXY motif are often required for complete interaction. The PID domain-containing proteins which bind APP require the YENPTY motif for full interaction. These interactions are independent of phosphorylation on the terminal tyrosine residue. The NPXY site is also involved in clathrin-mediated endocytosis.,Function:Appicans elicit adhesion of neural cells to the extracellular matrix and may regulate neurite outgrowth in the brain.,Function:Beta-amyloid peptides are lipophilic metal chelators with metal-reducing activity. Bind transient metals such as copper, zinc and iron. In vitro, can reduce Cu(2+) and Fe(3+) to Cu(+) and Fe(2+), respectively. Beta-amyloid 42 is a more effective reductant than beta-amyloid 40. Beta-amyloid peptides bind to lipoproteins and apolipoproteins E and J in the CSF and to HDL particles in plasma, inhibiting metal-catalyzed oxidation of lipoproteins. Beta-APP42 may activate mononuclear phagocytes in the brain and elicit inflammatory responses. Promotes both tau aggregation and TPK II-mediated phosphorylation. Interaction with overexpressed HADH2 leads to oxidative stress and neurotoxicity.,Function:Functions as a cell surface receptor and performs physiological functions on the surface of neurons relevant to neurite growth, neuronal adhesion and axonogenesis. Involved in cell mobility and transcription regulation through protein-protein interactions. Can promote transcription activation through binding to APBB1-HTATIP and inhibits Notch signaling through interaction with Numb. Couples to apoptosis-inducing pathways such as those mediated by G(O) and JIP. Inhibits G(o) alpha ATPase activity (By similarity). Acts as a kinesin I membrane receptor, mediating the axonal transport of beta-secretase and presenilin 1. Involved in copper homeostasis/oxidative stress through copper ion reduction. In vitro, copper-metallated APP induces neuronal death directly or is potentiated through Cu(2+)-mediated low-density lipoprotein oxidation. Can regulate neurite outgrowth through binding to components of the extracellular matrix such as heparin and collagen I and IV. The splice isoforms that contain the BPTI domain possess protease inhibitor activity.,Function:The gamma-CTF peptides as well as the caspase-cleaved peptides, including C31, are potent enhancers of neuronal apoptosis.,induction:Increased levels during neuronal differentiation.,mass spectrometry: PubMed:12214090,miscellaneous:Chelation of metal ions, notably copper, iron and zinc, can induce histidine-bridging between beta-amyloid molecules resulting in beta-amyloid-metal aggregates. The affinity for copper is much higher than for other transient metals and is increased under acidic conditions. Extracellular zinc-binding increases binding of heparin to APP and inhibits collagen-binding.,online information:Amyloid beta entry,online information:APP mutations,PTM:Extracellular binding and reduction of copper, results in a corresponding oxidation of Cys-144 and Cys-158, and the formation of a disulfide bond. In vitro, the APP-Cu(+) complex in the presence of hydrogen peroxide results in an increased production of beta-amyloid-containing peptides.,PTM:N- and O-glycosylated. O-linkage of chondroitin sulfate to the L-APP isoforms produces the APP proteoglycan core proteins, the appicans. The chondroitin sulfate chain of appicans contains 4-O-sulfated galactose in the linkage region and chondroitin sulfate E in the repeated disaccharide region.,PTM:Phosphorylation in the C-terminal on tyrosine, threonine and serine residues is neuron-specific. Phosphorylation can affect APP processing, neuronal differentiation and interaction with other proteins. Phosphorylated on Thr-743 in neuronal cells by Cdc5 kinase and Mapk10, in dividing cells by Cdc2 kinase in a cell-cycle dependent manner with maximal levels at the G2/M phase and, in vitro, by GSK-3-beta. The Thr-743 phosphorylated form causes a conformational change which reduces binding of Fe65 family members. Phosphorylation on Tyr-757 is required for SHC binding. Phosphorylated in the extracellular domain by casein kinases on both soluble and membrane-bound APP. This phosphorylation is inhibited by heparin.,PTM:Proteolytically cleaved by caspases during neuronal apoptosis. Cleavage at Asp-739 by either caspase-6, -8 or -9 results in the production of the neurotoxic C31 peptide and the increased production of beta-amyloid peptides.,PTM:Proteolytically processed under normal cellular conditions. Cleavage by alpha-secretase or alternatively by beta-secretase leads to generation and extracellular release of soluble APP peptides, S-APP-alpha and S-APP-beta, respectively, and the retention of corresponding membrane-anchored C-terminal fragments, C83 and C99. Subsequent processing of C83 by gamma-secretase yields P3 peptides. This is the major secretory pathway and is non-amyloidogenic. Alternatively, presenilin/nicastrin-mediated gamma-secretase processing of C99 releases the amyloid beta proteins, amyloid-beta 40 (Abeta40) and amyloid-beta 42 (Abeta42), major components of amyloid plaques, and the cytotoxic C-terminal fragments, gamma-CTF(50), gamma-CTF(57) and gamma-CTF(59).,sequence caution:Contamination by an Alu repeat.,similarity:Belongs to the APP family.,similarity:Contains 1 BPTI/Kunitz inhibitor domain.,subcellular location:Cell surface protein that rapidly becomes internalized via clathrin-coated pits. During maturation, the immature APP (N-glycosylated in the endoplasmic reticulum) moves to the Golgi complex where complete maturation occurs (O-glycosylated and sulfated). After alpha-secretase cleavage, soluble APP is released into the extracellular space and the C-terminal is internalized to endosomes and lysosomes. Some APP accumulates in secretory transport vesicles leaving the late Golgi compartment and returns to the cell surface. Gamma-CTF(59) peptide is located to both the cytoplasm and nuclei of neurons. It can be translocated to the nucleus through association with APBB1 (Fe65). Beta-APP42 associates with FRPL1 at the cell surface and the complex is then rapidly internalized. APP sorts to the basolateral surface in epithelial cells. During neuronal differentiation, the Thr-743 phosphorylated form is located mainly in growth cones, moderately in neurites and sparingly in the cell body. Casein kinase phosphorylation can occur either at the cell surface or within a post-Golgi compartment.,subunit:Binds, via its C-terminus, to the PID domain of several cytoplasmic proteins, including APBB family members, the APBA family, MAPK8IP1, SHC1 and, Numb and Dab1 (By similarity). Binding to Dab1 inhibits its serine phosphorylation (By similarity). Also interacts with GPCR-like protein BPP, FPRL1, APPBP1, IB1, KNS2 (via its TPR domains) (By similarity), APPBP2 (via BaSS) and DDB1. In vitro, it binds MAPT via the MT-binding domains (By similarity). Associates with microtubules in the presence of ATP and in a kinesin-dependent manner (By similarity). Interacts, through a C-terminal domain, with GNAO1. Amyloid beta-42 binds CHRNA7 in hippocampal neurons. Beta-amyloid associates with HADH2. Soluble APP binds, via its N-terminal head, to FBLN1. Interacts with CPEB1 (By similarity). Interacts with ANKS1B.,tissue specificity:Expressed in all fetal tissues examined with highest levels in brain, kidney, heart and spleen. Weak expression in liver. In adult brain, highest expression found in the frontal lobe of the cortex and in the anterior perisylvian cortex-opercular gyri. Moderate expression in the cerebellar cortex, the posterior perisylvian cortex-opercular gyri and the temporal associated cortex. Weak expression found in the striate, extra-striate and motor cortices. Expressed in cerebrospinal fluid, and plasma. Isoform APP695 is the predominant form in neuronal tissue, isoform APP751 and isoform APP770 are widely expressed in non-neuronal cells. Isoform APP751 is the most abundant form in T-lymphocytes. Appican is expressed in astrocytes.,
展开内容
细胞定位:
Cell membrane ; Single-pass type I membrane protein . Membrane ; Single-pass type I membrane protein . Perikaryon . Cell projection, growth cone . Membrane, clathrin-coated pit . Early endosome . Cytoplasmic vesicle . Cell surface protein that rapidly becomes internalized via clathrin-coated pits. Only a minor proportion is present at the cell membrane; most of the protein is present in intracellular vesicles (PubMed:20580937). During maturation, the immature APP (N-glycosylated in the endoplasmic reticulum) moves to the Golgi complex where complete maturation occurs (O-glycosylated and sulfated). After alpha-secretase cleavage, soluble APP is released into the extracellular space and the C-terminal is internalized to endosomes and lysosomes. Some APP accumulates in secretory transport vesicles leaving the late Golgi compartment and returns to the cell surface. APP sorts to the basolateral surface in epithelial cells. During neuronal differentiation, the Thr-743 phosphorylated form is located mainly in growth cones, moderately in neurites and sparingly in the cell body (PubMed:10341243). Casein kinase phosphorylation can occur either at the cell surface or within a post-Golgi compartment. Associates with GPC1 in perinuclear compartments. Colocalizes with SORL1 in a vesicular pattern in cytoplasm and perinuclear regions. .; [C83]: Endoplasmic reticulum . Golgi apparatus . Early endosome .; [C99]: Early endosome .; [Soluble APP-beta]: Secreted .; [Amyloid-beta protein 42]: Cell surface. Associates with FPR2 at the cell surface and the complex is then rapidly internalized. .; [Gamma-secretase C-terminal fragment 59]: Nucleus . Cytoplasm . Located to both the cytoplasm and nuclei of neurons. It can be translocated to the nucleus through association with APBB1 (Fe65) (PubMed:11544248). In dopaminergic neurons, the phosphorylated Thr-743 form is localized to the nucleus (By similarity). .
展开内容
组织表达:
Expressed in the brain and in cerebrospinal fluid (at protein level) (PubMed:2649245). Expressed in all fetal tissues examined with highest levels in brain, kidney, heart and spleen. Weak expression in liver. In adult brain, highest expression found in the frontal lobe of the cortex and in the anterior perisylvian cortex-opercular gyri. Moderate expression in the cerebellar cortex, the posterior perisylvian cortex-opercular gyri and the temporal associated cortex. Weak expression found in the striate, extra-striate and motor cortices. Expressed in cerebrospinal fluid, and plasma. Isoform APP695 is the predominant form in neuronal tissue, isoform APP751 and isoform APP770 are widely expressed in non-neuronal cells. Isoform APP751 is the most abundant form in T-lymphocytes. Appican is expressed in astrocytes.
展开内容
研究领域:
>>Serotonergic synapse ;
>>Alzheimer disease ;
>>Pathways of neurodegeneration - multiple diseases
>>Alzheimer disease ;
>>Pathways of neurodegeneration - multiple diseases
展开内容
信号通路
文献引用({{totalcount}})
货号: YT0226
规格
价格
货期
数量
200μL
¥3,780.00
现货
0
100μL
¥2,300.00
现货
0
40μL
¥960.00
现货
0
加入购物车


已收藏


收藏
Recently Viewed Products
Clear allToggle night Mode
{{pinfoXq.title || ''}}
Catalog: {{pinfoXq.catalog || ''}}
Filter:
All
{{item.name}}
{{pinfo.title}}
-{{pinfo.catalog}}
主要信息
Target
{{pinfo.target}}
Reactivity
{{pinfo.react}}
Applications
{{pinfo.applicat}}
Conjugate/Modification
{{pinfo.coupling}}/{{pinfo.modific}}
MW (kDa)
{{pinfo.mwcalc}}
Host Species
{{pinfo.hostspec}}
Isotype
{{pinfo.isotype}}
产品 {{index}}/{{pcount}}
上一个产品
下一个产品
{{pvTitle}}
滚轮缩放图片
{{pvDescr}}