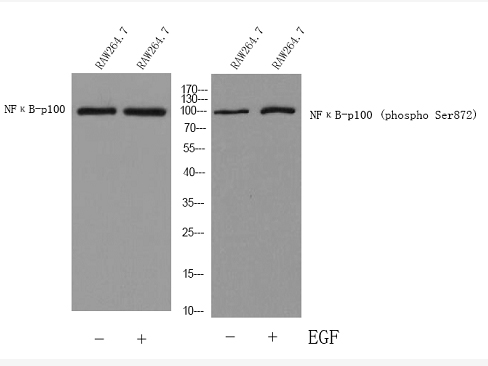
Total NF-κB p100/p52 Cell-Based Colorimetric ELISA Kit
- Catalog No.:KA4197C
- Applications:ELISA
- Reactivity:Human;Mouse;Rat
- Gene Name:
- NFKB2
- Human Gene Id:
- 4791
- Human Swiss Prot No:
- Q00653
- Mouse Swiss Prot No:
- Q9WTK5
- Storage Stability:
- 2-8°C/6 months
- Other Name:
- Nuclear factor NF-kappa-B p100 subunit (DNA-binding factor KBF2) (H2TF1) (Lymphocyte translocation chromosome 10 protein) (Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2) (Oncogene Lyt-10) (Lyt10) [Cleaved into: Nuclear factor NF-kappa-B p52 subunit]
- Detection Method:
- Colorimetric
- Background:
- disease:A chromosomal aberration involving NFKB2 is found in a case of B-cell non Hodgkin lymphoma (B-NHL). Translocation t(10;14)(q24;q32) with IGHA1. The resulting oncogene is also called Lyt-10C alpha variant.,disease:A chromosomal aberration involving NFKB2 is found in a cutaneous T-cell leukemia (C-TCL) cell line. This rearrangement produces the p80HT gene which encodes for a truncated 80 kDa protein (p80HT).,disease:In B-cell leukemia (B-CLL) cell line, LB40 and EB308, can be found after heterogeneous chromosomal aberrations, such as internal deletions.,domain:The C-terminus of p100 might be involved in cytoplasmic retention, inhibition of DNA-binding by p52 homodimers, and/or transcription activation.,domain:The glycine-rich region (GRR) appears to be a critical element in the generation of p52.,function:NF-kappa-B is a pleiotropic transcription factor which is present in almost all cell types and is involved in many biological processed such as inflammation, immunity, differentiation, cell growth, tumorigenesis and apoptosis. NF-kappa-B is a homo- or heterodimeric complex formed by the Rel-like domain-containing proteins RELA/p65, RELB, NFKB1/p105, NFKB1/p50, REL and NFKB2/p52. The dimers bind at kappa-B sites in the DNA of their target genes and the individual dimers have distinct preferences for different kappa-B sites that they can bind with distinguishable affinity and specificity. Different dimer combinations act as transcriptional activators or repressors, respectively. NF-kappa-B is controlled by various mechanisms of post-translational modification and subcellular compartmentalization as well as by interactions with other cofactors or corepressors. NF-kappa-B complexes are held in the cytoplasm in an inactive state complexed with members of the NF-kappa-B inhibitor (I-kappa-B) family. In a conventional activation pathway, I-kappa-B is phosphorylated by I-kappa-B kinases (IKKs) in response to different activators, subsequently degraded thus liberating the active NF-kappa-B complex which translocates to the nucleus. In a non-canonical activation pathway, the MAP3K14-activated CHUK/IKKA homodimer phosphorylates NFKB2/p100 associated with RelB, inducing its proteolytic processing to NFKB2/p52 and the formation of NF-kappa-B RelB-p52 complexes. The NF-kappa-B heterodimeric RelB-p52 complex is a transcriptional activator. The NF-kappa-B p52-p52 homodimer is a transcriptional repressor. NFKB2 appears to have dual functions such as cytoplasmic retention of attached NF-kappa-B proteins by p100 and generation of p52 by a cotranslational processing. The proteasome-mediated process ensures the production of both p52 and p100 and preserves their independent function. p52 binds to the kappa-B consensus sequence 5'-GGRNNYYCC-3', located in the enhancer region of genes involved in immune response and acute phase reactions. p52 and p100 are respectively the minor and major form; the processing of p100 being relatively poor. Isoform p49 is a subunit of the NF-kappa-B protein complex, which stimulates the HIV enhancer in synergy with p65.,PTM:Constitutive processing is tightly suppressed by its C-terminal processing inhibitory domain, named PID, which contains the death domain.,PTM:Subsequent to MAP3K14-dependent serine phosphorylation, p100 polyubiquitination occurs then triggering its proteasome-dependent processing.,PTM:While translation occurs, the particular unfolded structure after the GRR repeat promotes the generation of p52 making it an acceptable substrate for the proteasome. This process is known as cotranslational processing. The processed form is active and the unprocessed form acts as an inhibitor (I kappa B-like), being able to form cytosolic complexes with NF-kappa B, trapping it in the cytoplasm. Complete folding of the region downstream of the GRR repeat precludes processing.,similarity:Contains 1 death domain.,similarity:Contains 1 RHD (Rel-like) domain.,similarity:Contains 7 ANK repeats.,subcellular location:Nuclear, but also found in the cytoplasm in an inactive form complexed to an inhibitor (I-kappa-B).,subunit:Component of the NF-kappa-B RelB-p52 complex. Homodimer; component of the NF-kappa-B p52-p52 complex. Component of the NF-kappa-B p65-p52 complex. Component of the NF-kappa-B p52-c-Rel complex. NFKB2/p52 interacts with NFKBIE. Component of a complex consisting of the NF-kappa-B p50-p50 homodimer and BCL3.,
- Function:
- cell activation, adaptive immune response, follicular dendritic cell activation, follicular dendritic cell differentiation,adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains, germinal center formation, immune system development, transcription, regulation of transcription, DNA-dependent, immune response, extracellular matrix organization, extracellular structure organization, regulation of transcription, hemopoietic or lymphoid organ development, spleen development, regulation of RNA metabolic process,
- Subcellular Location:
- Nucleus. Cytoplasm. Nuclear, but also found in the cytoplasm in an inactive form complexed to an inhibitor (I-kappa-B).
- June 19-2018
- WESTERN IMMUNOBLOTTING PROTOCOL
- June 19-2018
- IMMUNOHISTOCHEMISTRY-PARAFFIN PROTOCOL
- June 19-2018
- IMMUNOFLUORESCENCE PROTOCOL
- September 08-2020
- FLOW-CYTOMEYRT-PROTOCOL
- May 20-2022
- Cell-Based ELISA│解您多样本WB检测之困扰
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-ACETYL-PROTEIN
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-PHOSPHO-PROTEIN
- July 13-2018
- Antibody-FAQs