- Home
- About
- Promotions
-
Products
-
Elisa Kits
- |
-
Primary antibodies
- |
-
Secondary antibodies
- |
-
Proteins
- |
-
IHC reagents
- |
-
WB reagents
- PonceauS Staining Solution
- PBST Washing Buffer, 10X
- 1.5M Tris-HCl Buffer, pH8.8
- 1M Tris-HCl Buffer, pH6.8
- 10% SDS Solution
- Prestained Protein Marker
- TBST Washing Buffer, 10X
- SDS PAGE Loading Buffer, 5X
- Stripping Buffered Solution
- Tris Buffer, pH7.4, 10X
- Total Protein Extraction Kit
- Running Buffer, 10X
- Transfer Buffer, 10X
- 30% Acr-Bis(29:1) Solution
- Tris电泳液速溶颗粒
- PBS(1X, premixed powder)
- TBS(1X, premixed powder)
- 快速封闭液
- 转膜液速溶颗粒
- Chemical reagents
- News
- Distributor
- Resources
- Contact
- Home
- >
- Info
- >
- p27 protein
- >
- Go Back
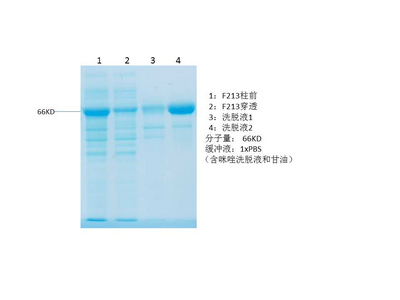
p27 protein
- Catalog No.:YD0077
- Applications:WB;SDS-PAGE
- Reactivity:Human
- Protein Name:
- p27 protein
- Sequence:
- Amino acid: 123-198, with his-MBP tag.
- Formulation:
- Liquid in PBS
- Concentration:
- SDS-PAGE >90%
- Storage Stability:
- -20°C/6 month,-80°C for long storage
- Other Name:
- Cyclin-dependent kinase inhibitor 1B (Cyclin-dependent kinase inhibitor p27) (p27Kip1)
- Background:
- disease:Defects in CDKN1B are the cause of multiple endocrine neoplasia type 4 (MEN4) [MIM:610755]. Multiple endocrine neoplasia (MEN) syndromes are inherited cancer syndromes of the thyroid. MEN4 is a MEN-like syndrome with a phenotypic overlap of both MEN1 and MEN2.,domain:A peptide sequence containing only AA 28-79 retains substantial Kip1 cyclin A/CDK2 inhibitory activity.,function:Important regulator of cell cycle progrssion. Involved in G1 arrest. Potent inhibitor of cyclin E- and cyclin A-CDK2 complexes. Positive regulator of cyclin D-dependent kinases such as CDK4. Regulated by phosphorylation and degradation events.,induction:Maximal levels in quiescence cells and early G(1). Levels decrease after mitogen stimulation as cells progress toward S-phase.,miscellaneous:Decreased levels of p27Kip1, mainly due to proteosomal degradation, are found in various epithelial tumors originating from lung, breast, colon, ovary, esophagus, thyroid and prostate.,PTM:Phosphorylated; phosphorylation occurs on serine, threonine and tyrosine residues. Phosphorylation on Ser-10 is the major site of phosphorylation in resting cells, takes place at the G(0)-G(1) phase and leads to protein stability. Phosphorylation on other sites is greatly enhanced by mitogens, growth factors, cMYC and in certain cancer cell lines. The phosphorylated form found in the cytoplasm is inactivate. Phosphorylation on Thr-198 is required for interaction with 14-3-3 proteins. Phosphorylation on Thr-187, by CDK2 leads to protein ubiquitination and proteasomal degradation. Tyrosine phosphorylation promotes this process. Phosphorylation by PKB/AKT1 can be suppressed by LY294002, an inhibitor of the catalytic subunit of PI3K. Phosphorylation on Tyr-88 and Tyr-89 has no effect on binding CDK2, but is required for binding CDK4. Dephosphorylated on tyrosine residues by G-CSF.,PTM:Ubiquitinated; in the cytoplasm by the KPC1/KPC2 complex and, in the nucleus, by SCF/SKP2. The latter requires prior phosphorylation on Thr-187.,similarity:Belongs to the CDI family.,subcellular location:Nuclear and cytoplasmic in quiescent cells. AKT- or RSK-mediated phosphorylation on Thr-198, binds 14-3-3, translocates to the cytoplasm and promotes cell cycle progression. Mitogen-activated UHMK1 phosphorylation on Ser-10 also results in translocation to the cytoplasm and cell cycle progression. Phosphorylation on Ser-10 facilitates nuclear export. Translocates to the nucleus on phosphorylation of Tyr-88 and Tyr-89.,subunit:Interacts with NUP50; the interaction leads to nuclear import and degradation of phosphorylated p27kip1. Interacts with COPS5, subunit of the COP9 signalosome complex; the interaction leads to p27KIP degradation. Interacts with SPDYA in the SPDYA/CDK2/p27kip1 complex. Interacts (Thr-198 phosphorylated-form) with 14-3-3 proteins, binds strongly YWHAQ, weakly YWHAE and YWHAH, but not YWHAB nor YWHAZ; the interaction with YWHAQ results in translocation to the cytoplasm. Interacts with AKT1, LYN and UHMK1; the interactions lead to cytoplasmic mislocation, phosphorylation of p27kip1 and inhibition of cell cycle arrest. Interacts (unphosphorylated form) with CDK2. Interacts (phosphorylated on Tyr-88 and Tyr-89) with CDK4; the interaction induces nuclear translocation. Interacts with GRB2.,tissue specificity:Expressed in all tissues tested. Highest levels in skeletal muscle, lowest in liver and kidney.,
- Function:
- regulation of cyclin-dependent protein kinase activity, G1/S transition of mitotic cell cycle, mitotic cell cycle, regulation of cell growth, regulation of transcription, DNA-dependent, negative regulation of protein kinase activity, ion transport,cation transport, potassium ion transport, induction of apoptosis, cell cycle, cell cycle arrest, sensory organ development, sensory perception, sensory perception of sound, cell death, positive regulation of cell proliferation,negative regulation of cell proliferation, regulation of cell size, negative regulation of biosynthetic process, negative regulation of macromolecule biosynthetic process, negative regulation of phosphorus metabolic process, negative regulation of macromolecule metabolic process, negative regulation of gene expression, positive regulation of organelle organization, regulation of cell death, positive regulation of cell de
- Subcellular Location:
- Nucleus. Cytoplasm. Endosome . Nuclear and cytoplasmic in quiescent cells. AKT- or RSK-mediated phosphorylation on Thr-198, binds 14-3-3, translocates to the cytoplasm and promotes cell cycle progression. Mitogen-activated UHMK1 phosphorylation on Ser-10 also results in translocation to the cytoplasm and cell cycle progression. Phosphorylation on Ser-10 facilitates nuclear export. Translocates to the nucleus on phosphorylation of Tyr-88 and Tyr-89. Colocalizes at the endosome with SNX6; this leads to lysosomal degradation (By similarity). .
- Expression:
- Expressed in kidney (at protein level) (PubMed:15509543). Expressed in all tissues tested (PubMed:8033212). Highest levels in skeletal muscle, lowest in liver and kidney (PubMed:8033212).