Anthrax Protective Antigen mouse mAb
- Catalog No.:YM1461
- Applications:ELISA
- Reactivity:Bacillus anthracis
- Target:
- Anthrax Protective Antigen
- Gene Name:
- pad4
- Human Gene Id:
- 3361714
- Human Swiss Prot No:
- P13423
- Immunogen:
- Specificity:
- This antibody detects Anthrax Protective Antigen.
- Formulation:
- Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
- Source:
- Monoclonal, Mouse
- Dilution:
- ELISA 1:10000-20000
- Purification:
- The antibody was affinity-purified from mouse ascites by affinity-chromatography using epitope-specific immunogen.
- Concentration:
- 1 mg/ml
- Storage Stability:
- -15°C to -25°C/1 year(Do not lower than -25°C)
- Other Name:
- anthrax PA;Anthrax toxins translocating protein;PA 83;PA;PA83;Pag;PagA;Protective antigen.
- Observed Band(KD):
- 86kD
- Background:
- P13423 pXO1-110(pxo1_110) Bacillus anthracis domain:The molecule is folded into four functional domains. Each domain is required for a particular step in the toxicity process. Domain 1 contains two calcium ions and the proteolytic activation site. Cleavage of the PA monomer releases the subdomain 1a, which is the N-terminal fragment of 20-kDa (PA20). The subdomain 1b is part of the remaining 63-kDa fragment (PA63) and contains the binding sites for LP and EF. Domain 2 is a beta-barrel core containing a large flexible loop that has been implicated in membrane insertion and pore formation. There is a chymotrypsin cleavage site in this loop that is required for toxicity. Domain 3 has a hydrophobic patch thought to be involved in protein-protein interactions. Domain 4 appears to be a separate domain and shows limited contact with the other three domains: it would swing out of the way during membrane insertion. It is required for binding to the receptor; the small loop is involved in receptor recognition.,function:One of the three proteins composing the anthrax toxin, the agent which infects many mammalian species and that may cause death. PA binds to a receptor (ATR) in sensitive eukaryotic cells, thereby facilitating the translocation of the enzymatic toxin components, edema factor and lethal factor, across the target cell membrane. PA associated with LF causes death when injected, PA associated with EF produces edema. PA induces immunity to infection with anthrax.,miscellaneous:In PubMed:10085028 multiple mutagenesis experiments were performed that showed that the residues present in the small loop of domain 4, and not the ones in the large loop, are involved in receptor recognition. In PubMed:14623961 high-throughput scanning mutagenesis experiments were performed in which all residues of PA-63 were mutated into Cys. Dominantly negative (DN) mutants were all clustered in domain 2. DN mutants prevent the conformational transition of PA-63 from the prepore to the pore state.,PTM:Proteolytic activation by furin or a furin-like protease cleaves the protein in two parts, PA-20 and PA-63; the latter is the mature protein. The cleavage occurs at the cell surface and probably in the serum of infected animals as well; both native and cleaved PA are able to bind to the cell receptor. The release of PA20 from the remaining receptor-bound PA63 exposes the binding site for EF and LF, and promotes oligomerization and internalization of the protein.,similarity:Belongs to the bacterial binary toxin family.,subcellular location:Secreted through the Sec-dependent secretion pathway. Therefore, PA is translocated across the membrane in an unfolded state and then it is folded into its native configuration on the trans side of the membrane, prior to its release to the environment. PA requires the extracellular chaperone prsA for efficient folding.,subunit:Anthrax toxins are composed of three distinct proteins, a protective antigen (PA), a lethal factor (LF) and an edema factor (EF). None of these is toxic by itself. PA+LF forms the lethal toxin (LeTx); PA+EF forms the edema toxin (EdTx). PA-63 forms heptamers and this oligomerization is required for LF or EF binding. This complex is endocytosed by the host. Once activated, at low pH, the heptamer undergoes conformational changes and converts from prepore to pore inserted in the membrane, forming cation-selective channels and triggering the release of LF and EF in the host cytoplasm.,
- Function:
- P13423 pXO1-110(pxo1_110) Bacillus anthracis domain:The molecule is folded into four functional domains. Each domain is required for a particular step in the toxicity process. Domain 1 contains two calcium ions and the proteolytic activation site. Cleavage of the PA monomer releases the subdomain 1a, which is the N-terminal fragment of 20-kDa (PA20). The subdomain 1b is part of the remaining 63-kDa fragment (PA63) and contains the binding sites for LP and EF. Domain 2 is a beta-barrel core containing a large flexible loop that has been implicated in membrane insertion and pore formation. There is a chymotrypsin cleavage site in this loop that is required for toxicity. Domain 3 has a hydrophobic patch thought to be involved in protein-protein interactions. Domain 4 appears to be a separate domain and shows limited contact with the other three domains: it would swing out of the
- June 19-2018
- WESTERN IMMUNOBLOTTING PROTOCOL
- June 19-2018
- IMMUNOHISTOCHEMISTRY-PARAFFIN PROTOCOL
- June 19-2018
- IMMUNOFLUORESCENCE PROTOCOL
- September 08-2020
- FLOW-CYTOMEYRT-PROTOCOL
- May 20-2022
- Cell-Based ELISA│解您多样本WB检测之困扰
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-ACETYL-PROTEIN
- July 13-2018
- CELL-BASED-ELISA-PROTOCOL-FOR-PHOSPHO-PROTEIN
- July 13-2018
- Antibody-FAQs
- Products Images
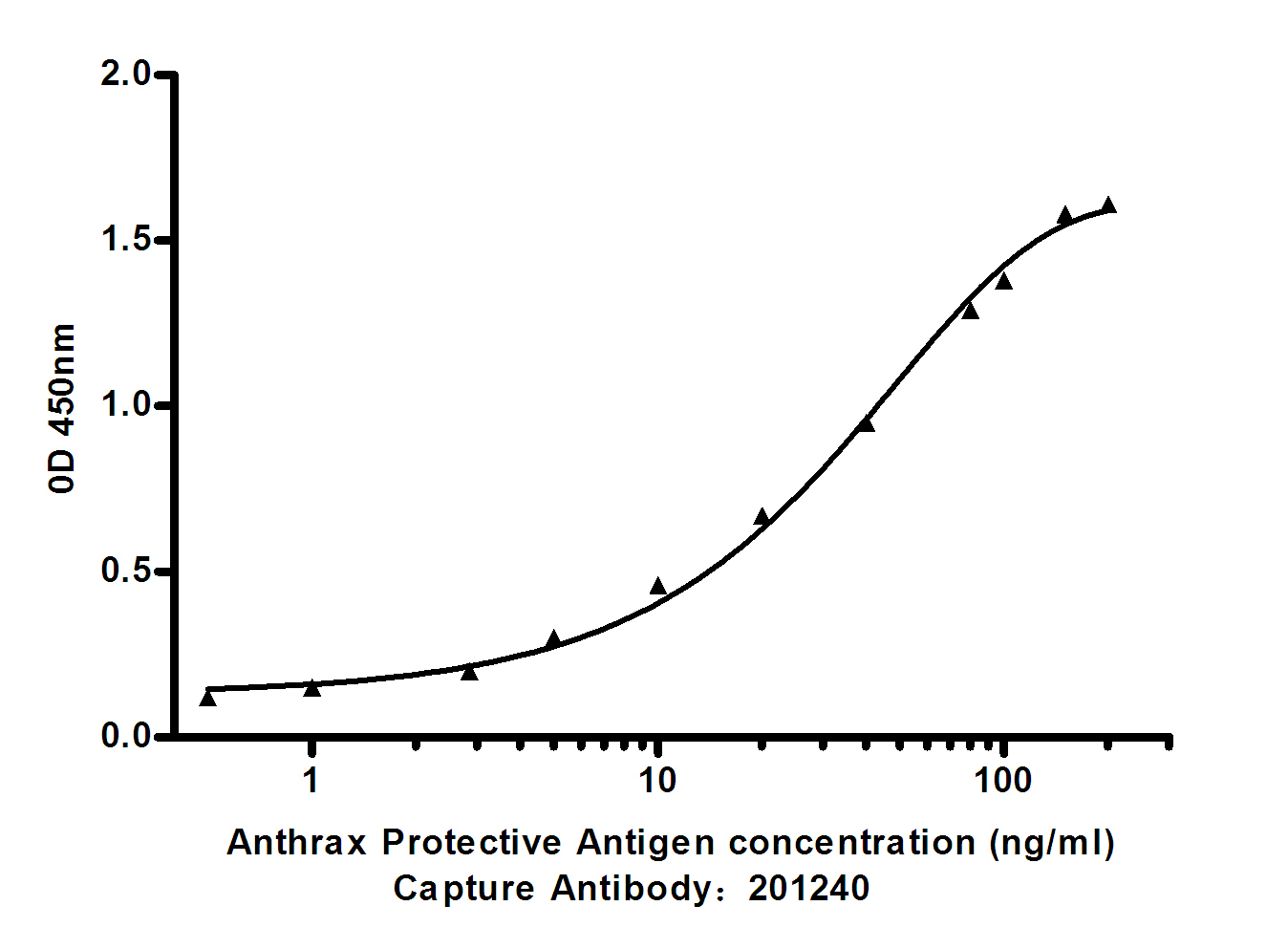
- Standard Curve for Anthrax Protective Antigen: Capture Antibody Mouse mAb 201240 to Anthrax Protective Antigen at 4μg/ml and other antibody for detecting.